- Home
- Catalogue
- Services
- Radiopharmacy
- Contact Us
- Sign in
DTPA Radpharm
DTPA 1990/1
Radpharm DTPA consists or sterile. pyrogen free,Jtapnmsed ingredients which need reconstitution with SOdium Pertecnnetate t"Tc) lfltCClion to produce a tectinetlum [IIIITl-rc) pentetate complex suital>te for renal imaging.
The precise structure of the technetium ("-le) pentetate compleK Is not known at tnls time.
Technetium j-.,.c] pentetate Is a diagnostic pharmaceutical administered by intravenous Injection.
PRODUCT INFORMATION
DTPA Radpharm Is supplied as a carton of 5 sterile, pyrogen free. vacuum
sealed Multi-dose 8 ml vials.
Each vial contains 5 mg pentetic acid and 1 mg stannous chloride anhydrous as a lyophilised powder.
The product contains no preservatives.
Technetium 99mTc Pentetate may be used as a renal perfusion imaging pharmaceutical.
GENERAL
Radiopharmaceuticals should be used only by physicians who are qualified by specific training in the safe use and handling of radionuclides.
Contents of the vial/s are intended only for use in the preparation of 99mTc pentetate.
The radioactivity of the dose should be checked with a suitable instrument immediately prior to administration.
Disposal of all radioactive wastes snouI0 be carried out in accordance with the NH & MRC "Code of Practice for me Disposal or Radioactive Wastes
by tile user"' (1985).
USE IN PREGNANCY
Tecnnetium-99m radiopharmaceuticals should only be given to a pregnant woman If in the judgement of the treating physician the expected benefits outweigh the potential hazards
USE DURING LACTATION
Tecnnellum-99m is excreted in human milk. Interruption to breast feeding Is necessary after the administration of 99mTc pentetate for a period less than 12hr.
(Reference: L.K. Harding, A. Bossuyt, s. Pellet, C. Remers, J.N. TaI001,
"Recommendations for nuclear medicine physicians regarding breastfeeding mothers", Eur.J.NUCI.Med., 1995, 22, BP17).
For each patient, exposure to ionising radiation must be justifiable on the basis or likely benefit. The activity administered must be such that the resulting dose is as low as reasonably achievable bearing in mind the need to obtain the intended diagnostic or therapeutic result.
Exposure to ionising radlation Is linked with cancer induction and a potential for development of hereditary defects. For diagnostic nuclear medicine investigations the current evidence suggests that these adverse effects will occur with low frequency because or the low radiation doses incurred.
For most diagnostic inves119ations using a nuclear medicine procedure the radiation dose delivered (EDE) Is less than 20 msv. Higher doses may be justified in some clinical circumstances.
In Isolated cases the following adverse reactions have been reported: flushing, dizziness. dyspnoea. itch, urticaria and hypotension.
ARTG entry for Medicine Registered
ARTG No: 14327
Sponsor: Global Medical Solutions Australia Pty Limited T/A Radpharm Scientific
Postal Address: PO Box 3334 BMDC, BELCONNEN, ACT, 2617 Australia
ARTG Start Date: 5/09/1991
Product Category: Medicine
Status: Active
Approval Area: Drug Safety Evaluation Branch
ARTG Summary:
Related Items
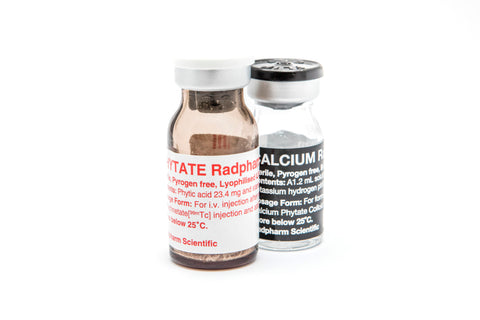
Calcium Phytate Colloid Radpharm
DESCRIPTION Kit for the Preparation of Technetium[99mTc] Calcium Phytate ColloidInjection for Liver/Spleen Imaging. The agent comprises sterile, pyrogen free lyophilised ingredients whichneed reconstitution with sodium... View full product details
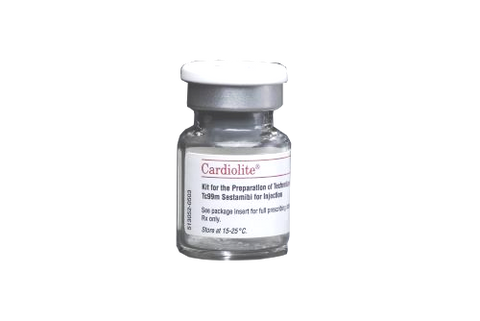
Cardolite Sestamibi
Cardiolite® is a technetium-labeled single photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) agent used for identifying and managing patients with known or suspected... View full product details
Definity
DESCRIPTION Cardiolite® is a technetium-labeled single photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) agent used for identifying and managing patients with known or... View full product details
DMSA Radpharm
Radpharm DMSA consists of sterile, pyrogen free lyophilised ingredients which need reconstitution with sodium pertechnetate[99mTc] injection to produce a technetium[99mTc] succimer complex suitable for renal imaging.The... View full product details
Sign up to get the latest on sales, new releases and more …
© 2025 GMS Australia Pty Ltd.
PRODUCTS NOT AVAILABLE FOR PURCHASE BY THE GENERAL PUBLIC .ALWAYS READ THE LABEL AND FOLLOW THE DIRECTIONS FOR USE
Powered by Shopify